Cambridge Cardiovascular / BHF Cambridge CRE annual symposium 2024
Browse abstracts or skip to listing by theme:
| POPULATION AND DATA SCIENCE | |
| 1 | COVID-19 and vascular and inflammatory diseases: cohort study using linked electronic health records on over 14 million children and young people in England. |
| Alexia Sampri, Wen Shi, Genevieve Cezard, Thomas Bolton, Rochelle Knight, Venexia Walker, Rachel Denholm, Jonathan Sterne, William Whiteley, Angela Wood | |
| Background The extent to which COVID-19 diagnosis and vaccination are associated with risks of vascular and inflammatory diseases in under 18-year-olds remains uncertain. Methods We studied England-wide linked electronic health records in up to 14,453,460 under 18-year-olds from 1 Jan 2020 to 31 December 2022. We calculated adjusted hazard ratios (HRs) for vascular and inflammatory diseases according to the time since COVID-19 diagnosis and vaccination. Results We recorded 3,928,245 COVID-19 diagnoses, 2,310 arterial thrombotic events, 5,220 venous thrombotic events, 3,535 thrombocytopenia events, 2,045 myocarditis/pericarditis events, and 3,010 inflammatory conditions. COVID-19 diagnosis was associated with higher risks of venous thromboembolism (adjusted HR 2.84, 95% CI 2.33-3.46), thrombocytopenia (2.08, 1.47-2.94), myocarditis/pericarditis (1.95, 1.35-2.81), and inflammatory conditions (7.51, 6.02-9.35) in weeks 1-4 after diagnosis, which remained elevated up to 6 months after a diagnosis. In 9,245,395 children/young people aged 5-17 years who were eligible for vaccination, 3,407,560 received their first Pfizer vaccine. There was a higher incidence of myocarditis/pericarditis in weeks 1-4 after vaccine (1.84, 1.25-2.72). Conclusions Children and young people with a COVID-19 diagnosis have higher risks of vascular and inflammatory diseases up to 6 months after a diagnosis. We observed higher rates of myocarditis/pericarditis after first Pfizer vaccine in children and young people, as also recognised for adults. | |
| 2 | The socioeconomic trajectories of early adulthood and associations with cardiometabolic health at age 24 years |
| Eleanor Winpenny, Jan Stochl, Alun Hughes, Laura Howe, Kate Tilling | |
| Socioeconomic factors are strongly associated with cardiovascular outcomes. However, little is known about the immediate health impacts of socioeconomic exposures during early adulthood. In this study we identify different socioeconomic trajectories of early adulthood (age 16-24y), and assess associations with cardiometabolic health at age 24y. Methods ALSPAC cohort participants with education and employment data across ages 16-24y were included (n=7,568). Longitudinal latent class analysis identified classes following different early adulthood socioeconomic trajectories, based on participation in education and employment, education level attained and occupational class. Cardiometabolic outcomes included anthropometric measures, metabolic biomarkers, and structural cardiovascular measures. We modelled differences in outcomes across socioeconomic trajectory classes, adjusting for sex, ethnicity, childhood socioeconomic position, adolescent health behaviours and adolescent health. Results We identified four classes of early adulthood socioeconomic trajectory: (1) Higher Education (41% of the population), (2) Late Education (9%), (3) Part-Time Employment (21%), and (4) Early Employment (29%). Late Education was found to be the healthiest class, with Early Employment the least healthy class, although some differences in patterns were seen across outcomes. For example, estimated percentage body fat was 34.6% (95%CI:34.0-35.2%) in the Higher Education class, which was similar in the Late Education class (-0.3%, p=0.53) but higher in Early Employment (1.4%, p= 0.003) and Part-Time Employment (1.4%, p=0.01) classes. Conclusion Identification of socioeconomic trajectories provides a person-centred method for assessment of socioeconomic exposures across the period of early adulthood. These trajectories contribute to inequalities in cardiovascular health, which can already be identified at age 24y. | |
| 3 | Can telephone training improve ECG quality in remote screening for atrial fibrillation? |
| Kethaki Prathivadi Bhayankaram, Jonathan Mant, James Brimicombe, Andrew Dymond, Kate Williams and Peter H. Charlton | |
| Background and Aims: Self-recorded, single-lead ECGs are increasingly used to diagnose arrhythmias. However, they can be of variable quality, which can affect the reliability of interpretation. In this analysis of ECGs collected in atrial fibrillation screening studies, our aims were to: (i) determine the quality of ECGs when recorded unsupervised (at home); and (ii) investigate whether telephone training improved ECG quality. Methods: Data was obtained from the Screening for Atrial Fibrillation to Reduce stroke (SAFER) programme, where participants recorded single-lead ECGs four times per day for three weeks using a handheld device. ECG quality was assessed using an automated algorithm, and participants who recorded >25% poor quality ECGs from days 4-10 of screening were identified for training. Telephone training to improve ECG recording technique was delivered when research team capacity permitted. Results: 14,727 participants recorded 1,206,972 ECGs, of which 43,513 (3.6%) were poor quality. Most participants (51.9%) did not record any poor-quality ECGs. 1,105 (7.5%) participants met the threshold for training. Of these, 165 participants received training and 896 did not. Comparing these groups, the mean (95% confidence interval) reduction in the proportion of poor-quality ECGs per participant from before training (days 1-3) to after training (days 11-21) was 21.1 (17.5-23.5) % with training and 15.7 (14.5-16.8) % without training (p<0.05). Conclusion: Most participants achieved adequate quality ECGs. For those that did not, ECG quality improved over time regardless, and training further improved ECG quality. Therefore, telephone training could be considered in atrial fibrillation screening programmes using single-lead ECG devices. | |
| 4 | Using metabolomics to identify novel risk factors for cerebral small vessel disease and vascular dementia to prioritise potential therapeutic targets |
| Wen Hui Ng, Professor Hugh Markus, Dr Eric Harshfield | |
| Background: Despite cerebral small vessel disease (SVD) being a major cause of ischemic stroke and vascular dementia, its genetic basis and underlying pathogenesis are currently not well understood due to the difficulty in investigating such small vessels in vivo and the lack of attention given to cerebral SVD as an important mechanism in stroke until recently. The increased availability of large genetic, metabolomic and clinical databases such as UK Biobank, however, presents the opportunity to obtain a more comprehensive understanding of the pathophysiology of cerebral SVD through bioinformatics. Methods: We analysed metabolomic and clinical data from the DNA Lacunar 1 & 2 studies to identify novel risk factors for cerebral SVD and vascular dementia. Linear regression analyses adjusted for age and sex were performed to identify whether certain metabolites lead to changes in severity of cerebral SVD or risk of lacunar stroke. Results: Observational analyses showed that total cholesterol (including LDL-C and HDL-C), phosphatidyl, glycA, histidine and phenylalanine were associated with SVD imaging traits including number of microbleeds, number of lacunes and white matter hyperintensities volume. ApoB, cholesterol and unsaturated fatty acids were associated with lacunar stroke. Discussion: Initial findings suggest that metabolites may be useful in predicting risk of lacunar stroke. We are in the process of conducting further analyses using Mendelian randomization to determine whether the observed associations are likely to be causal. This could help uncover important risk factors of cerebral SVD and guide therapeutic intervention. | |
| 5 | Detailed and up-to-date estimates of cardiovascular disease burden: aims and methods of a whole-population study in England |
| Elias Allara, Wen Shi, Elena Raffetti, Tom Bolton, Genevieve Cezard, Spiros Denaxas, Angela Wood, William Whiteley | |
| Assessing the burden of cardiovascular diseases in recent years in England poses significant challenges, hindering the evaluation of COVID-19's impact on key cardiovascular conditions such as stroke, myocardial infarction, heart failure, and peripheral vascular disease, as well as less common cardiovascular conditions. Currently, cause-specific mortality estimates from the Office for National Statistics and some epidemiological metrics of selected cardiovascular conditions from the Office for Health Improvement and Disparities are available at the national level. These estimates may not always be accessible at the regional level, or for key subgroups such as those defined by deprivation and ethnicity, and may not necessarily include less common cardiovascular conditions. Additionally, while more detailed analyses can be performed at the level of individual healthcare organisation, this approach may lead to fragmentation and methodological heterogeneity. We aim to leverage linked whole population electronic health records from >50M individuals available in the NHS England Secure Data Environment to: i. define >50 clinically recognised cardiovascular conditions using information from general practice, hospital data and specialist registers; ii. estimate major epidemiological metrics (incidence, prevalence, recurrence rate and case fatality) for these conditions at the national level, and for four key conditions (stroke, myocardial infarction, heart failure and peripheral vascular disease) at both national and regional level; iii. present detailed subgroup analyses by age, sex, deprivation, multi-morbidity and ethnicity. We expect these estimates to be influential for healthcare planning, research and the third sector by revealing hidden inequalities across services, regions, and socio-demographic characteristics. | |
| FUNCTIONAL GENOMICS | |
| 6 | Deconvoluting genomic variants in thoracic aortic disease using iPSC models |
| Aminder A. Singh, Deeti K. Shetty, Semih Bayraktar, Aishwarya G. Jacob, Liviu Pirvan, Irina Mohorianu, Sanjay Sinha | |
| Background A critical challenge in genomic medicine is determining which variants identified by whole genome sequencing are causal for disease. Induced pluripotent stem cell (iPSC) models of aortic vascular smooth muscle cells (VSMC) recapitulate key disease features and provide a good, humanised model of the cellular changes that lead to aortic wall failure. Aim To use iPSC models of thoracic aortic diseases (TAD) to analyse Tier 1 genetic variants identified in the 100,000 Genomes Project. Methods Genetic variants in SMAD4 (51076665 C>T), MYLK (123401069 ACT>A) and LOX (122070160 T>TA) were selected. Using CRISPR-Cas9, these variants were knocked into a wild-type iPSC line (HPSI0114-eipl_3). The mutant lines were differentiated into VSMCs via neural crest lineage. Bulk mRNAseq was performed after 12 days of VSMC differentiation and further 14 days of maturation in serum free medium. Results TAD variant heterozygous knock ins were confirmed through molecular cloning and sequencing. All mutants demonstrated TAD transcriptomic phenotypes with common abnormalities in apoptosis, the extracellular matrix, cytoskeleton, and contractile machinery. Several targetable signalling pathways were identified. Furthermore, distinctly modulated pathways were identified in individual variants, such as perturbed calcium signalling in MYLK mutant, or an inflammatory phenotype strongly associated with SMAD4 mutant. Mutant VSMCs were more proliferative and migratory suggestive of synthetic phenotype switching. Conclusion iPSCs derived VSMCs recapitulate TAD transcriptomic signatures and can be used to model TAD genetic variants. They provide a platform to undertake translational work with drug testing and build complex 3D perfusable coculture models. | |
| 7 | The joint impact of age, sex and genetics on multi-omic traits |
| Catherine Michelutti, Yu Xu, Michael Inouye | |
| Genetic predictions of multi-omic traits have become important tools for understanding the molecular and biological mechanisms underlying complex diseases. It has been established that age and sex can play a significant role in modifying the genetic risk of individuals for complex diseases. However, their potential impact in the context of multi-omic genetic predictions remains an area of ongoing investigation. This study utilises multi-omics profiled in two population studies (INTERVAL and UK Biobank) to examine the impact of age and sex on the predictability of multi-omic genetic scores. Using the INTERVAL-trained genetic scores for multi-omic traits, this study reveals scores with age- and sex-specific effects in the UKB and pinpoints the genetic variants attributed to these specific effects. Furthermore, this study examines the contributions of genetic components with age and/or sex-specific impact to the polygenic risk of complex diseases, elucidating their role in modulating genetic susceptibility across various diseases and traits through these biomolecular markers. | |
| 8 | The AZ-MRC-MTI Functional Genomics Screening Laboratory |
| Nicola McCarthy, Erica Bello and Ulrike Künzel | |
| The Milner Therapeutics Institute has entered a new partnership with AstraZeneca and the Medical Research Council (MRC) to establish a state-of-the-art arrayed functional genomics laboratory, which will be housed at the Milner Therapeutics Institute (MTI). The Functional Genomics Screening Laboratory (FGSL), which is expected to become operational in early 2025, will become part of the UK’s Human Functional Genomics Initiative. This is a key commitment in the Life Sciences Vision to support world class research and innovation in functional genomics and will contribute to the UK’s ambition of having the most advanced genomic healthcare system in the world. The FGSL will provide researchers from across the UK with access to large-scale biological and technological tools and house an advanced automated arrayed-CRISPR screening platform. Data generated by the FGSL will provide insights into the relationship between genes and disease, enabling scientists to discover new opportunities to develop therapies for chronic diseases including cardiovascular, respiratory and metabolic disease. UK academics will be able to apply to have their human in vitro model of choice explored using CRISPR libraries, with library and screening costs covered for most applications. Submissions will be considered by a Joint Steering Committee and successful applicants will enter into a collaborative agreement with the MTI (University of Cambridge) and AstraZeneca to agree the parameters of the screen. All data will be owned by the collaborating academic. To learn more about this opportunity and how to apply, please visit our poster. | |
| 9 | Glucose-Lowering Drug Target Perturbation and Risk of Cerebral Small Vessel Disease: A Drug Target Mendelian Randomization Analysis |
| Wenjing Ge, Hui Hong, Hugh S. Markus and Eric L. Harshfield | |
| Background: Observational studies have yielded inconsistent results regarding the association between the use of glucose-lowering medications and the risk of cerebral small vessel disease (cSVD). Leveraging naturally occurring genetic variations in genes responsible for glucose-lowering drug targets provides an opportunity to examine whether pharmacological perturbations are causally associated with the risk of cSVD. Methods: We performed a drug target Mendelian randomization analysis to assess the impact of genetically proxied perturbations of glucose-lowering drug targets on the risk of cSVD in the general population. Results: Genetically proxied perturbation of metformin targets was associated with lower peak width of skeletonized mean diffusivity (PSMD; beta -1.023 [-1.979, -0.248]) and lower risk of extensive hippocampal perivascular space (PVS) burden (OR 0.482 [0.274, 0.883]). In contrast, genetically proxied perturbation of sulfonylureas was not associated with imaging markers of cSVD. Genetically proxied perturbation of acarbose was the only glucose-lowering drug that was associated with higher risk of extensive white matter PVS burden (1.016 [1.003, 1.028]) and lobar cerebral microbleeds (1.287 [1.014, 1.634]). No associations were identified between genetic variations in the target gene regions of the three glucose-lowering drugs and any other outcome, including fractional anisotropy, white matter hypertension, basal ganglia PVS, and lacunar stroke. Conclusions: Current evidence supporting clinical trials that focus on the primary prevention of extensive hippocampal PVS burden and PSMD with metformin remains weak. However, our study emphasizes the prioritization of metformin-related genes as potential drug targets to reduce risk of extensive hippocampal PVS or higher PSMD in the general population. | |
| 10 | Inflammatory profiling informs mechanisms and treatment of cerebral small vessel disease |
| Zihan Sun, Eric Harshfield, Ziad Mallat, Niels P. Riksen, Stephen Burgess, Frank-Erik de Leeuw, and Hugh Markus | |
| IMPORTANCE Inflammation has been implicated in cerebral small vessel disease (SVD), a debilitating neurological condition leading to stroke and dementia. However, whether it is causal, and if so which components of the inflammatory pathways represent potential treatment targets remains uncertain. OBJECTIVE To identify circulating protein markers of inflammation associated with SVD. METHODS The association between inflammation and SVD was tested by two-sample Mendelian Randomization (MR) with genetic instruments derived mainly from the UK Biobank Pharma Proteomic Project (n = 34557). In total, 1010 inflammatory markers were examined in their associations with SVD, which was represented by both imaging-confirmed lacunar stroke [n=3199] and 4 MRI features of SVD (white matter hyperintensities [n=37355], perivascular space burden [n=38598], diffusion tensor imaging markers including fractional anisotropy [n=36533] and mean diffusivity [n=36460]). Colocalization analysis was used to identify genetic variants co-regulating each causal marker’s cell-type-specific expression, tissue-specific expression, blood concentration, and SVD. RESULTS In total, 4960 MR tests were performed based on the availability of genetic instruments. After multiple testing correction, TRIM21, a ubiquitin-protein ligase, showed significant association with lacunar stroke. Twelve additional markers were found to be associated with ≥1 MRI features of SVD. Colocalization analysis revealed that 4 of the 13 markers shared genetic loci co-regulating their circulating concentrations and SVD risk (TRIM21, METAP1D - a methionine aminopeptidase, EPHA2 - an ephrin receptor, APOE - apolipoprotein E). CONCLUSIONS Study findings are consistent with inflammation playing a causal role in disease pathogenesis. In current research, I am mapping the downstream cellular pathways to identify druggable targets | |
| CARDIOVASCULAR MEDICINE | |
| 11 | The early sprouts of the heart – Modelling human endocardium derived coronary plexus formation in organoid models |
| Beatrice Waller, Maria Köhne, Jonathan Lee, Alex Pascual, Vincent Knight-Schrijver, Sanjay Sinha | |
| Introduction: The inability of the adult heart to regenerate post-myocardial infarction is associated with its low angiogenic potential. The reactivation of molecular pathways that govern coronary plexus formation in development represents a promising avenue for novel therapeutics. However, there is still a lack of understanding regarding coronary network formation. In murine models, the endocardium, represents a major progenitor source of coronary endothelial cells (cECs). The signalling pathways that drive this endocardial to cEC differentiation and their relevance in human development remains unexplored due to lack of human fetal models. We aim to address these limitations through the establishment of a human embryonic stem cell (hESC)-derived organoid model of endocardial-cEC transition, recapitulating the early developmental niche in vitro. Methods and Results: We have successfully adapted an optimised hESC-Endocardium differentiation protocol developed by our laboratory into an organoid model (endocardioid). By varying the composition of growth factors including VEGF-A and WNT modulators we could instruct the differentiation of cardiomyocytes and endothelial cells, confirming their location using immunostaining. Our organoids self-organise with distinct cardiomyocyte layers and spontaneously contract. Furthermore, in a pilot sprouting assay we have demonstrated their potential capacity to transition to a more cEC-like fate with endothelial sprouts forming when exposed to pro-angiogenic VEGF-A. Conclusions: We show promising preliminary data for the generation of multicellular endocardioids containing multiple cardiac cell types. Future work will now focus on developing reporter cell lines for live cell tracking of hESC-Endocardium in our organoid platform and the identification of further signals that enhance sprouting angiogenesis. | |
| 12 | Can Minocycline Reduce Neuroinflammation and Blood-Brain Barrier Permeability in Cerebral Small Vessel Disease? |
| Daniel J Tozer, Robin B Brown, Laurence Loubière, Eric L Harshfield, Young T Hong, Tim D Fryer, Guy B Williams, Martin J Graves, Franklin Aigbirhio, John T O’Brien & Hugh S Markus | |
| Background: Cerebral small vessel disease is the major cause of vascular dementia but there are no effective treatments. Neuroinflammation may play a role in pathogenesis and in an animal model minocycline reduced both neuroinflammation and blood-brain barrier (BBB) permeability and improved outcome (Stroke 2014;45:1531–1538). We determined whether a similar effect could be replicated in man in the double-blind phase 2 MINERVA RCT (ISRCTN15483452). Methods: Forty-four subjects with SVD (lacunar stroke and white matter hyperintensities) were scanned simultaneously with contrast enhanced MRI for BBB permeability, and C11-PK11195 PET to assess microglial activation (“neuroinflammation”) at baseline, and after 3 months of minocycline (100mg twice daily) or placebo. Our co-primary endpoints were the volume of 11C-PK11195 binding hotspots and volume of BBB permeability ‘hotspots.’ Secondary endpoints were mean 11C-PK11195 binding and mean BBB permeability in the normal appearing white matter (NAWM), and serum inflammatory markers. Results: There was no treatment effect of minocycline on the change in percentage of 11C-PK11195 binding hotspots (RR 1.01, 95% CI 0.98–1.04), or on BBB permeability hotspots (RR 0.97, 95% CI 0.91–1.03). There was no effect of minocycline on mean 11C-PK11195 binding or mean BBB permeability, or on serum inflammation markers. Conclusions: In this RCT minocycline did not reduce microglial activation, or BBB permeability in lacunar stroke. We were unable to replicate results found in an animal model. Possible reasons might include differing doses, or that animal models of SVD do not reflect well the process in man. | |
| 13 | In-vitro model of hypertension by novel mutation in PPP1R12A (myosin phosphatase target subunit 1) |
| Deeti K Shetty, Anja S, S W Joyce Ho, Katriina Aalto-Setälä, Kaisa Ylänen, Pasi I Nevalainen and Sanjay Sinha | |
|
A 5-month old patient with bilateral coronary artery stenosis was found to have a de novo heterozygous point mutation in the PPP1R12A gene. This is a first report of a point-mutation in MyPhoNe (Myosin Phosphatase N-terminal Element) motif of PPP1R12A presenting with severe clinical features of cardiovascular involvement, ischemic heart failure and hypertension. The MyPhoNe motif mediates docking of regulatory proteins to the catalytic subunit of Myosin phosphatase enzyme PP1. To investigate the pathology, patient and healthy donor cells were reprogrammed to iPSCs followed by in vitro differentiation to vascular smooth muscle cells (vSMCs) for phenotypic and functional analyses. We validated the abnormal hypercontractile phenotype of mutant cells owing to enhanced myosin phosphorylation activity and correction of point-mutation using CRISPR-Cas9 ameliorated the heightened cellular tension to restore normal contractile function. Comparing hypercontractile vSMCs to normal cells holds the key towards understanding functional aspects of contractility and mechanical properties of vSMC in a range of vascular diseases, which remains underappreciated. Hence, we conducted proteomic analyses and cell-based phenotyping and found that our model of hypertensive-vSMC show marked upregulation of lipid biogenesis and accumulation. The use of antihypertensive medications Carvedilol and Nifedipine reduced cytoskeletal tension and normalised traction force. Further studies are warranted to investigate the link between increased SMC cytoskeletal tension and derailment of cellular processes that may predispose to cardiovascular disease. We hypothesise that antihypertensive drugs, in addition to reducing hypertension, could beneficially impact by regulating additional disease-associated pathways such as lipid biogenesis. |
|
| 14 | Mitochondrial dysfunction increases atherosclerosis by affecting T follicular helper cell differentiation |
| Despina Giakomidi , James Harrison, Peter Jones, Shuhong Chen, Katie Law, Leanne Masters, Nikki Figg, Jane Goodall, Josef Peninger, Ziad Mallat, Meritxell Nus | |
| The adaptive immune response plays an important role in atherosclerosis. We have previously demonstrated that Marginal Zone B (MZB) cells – T follicular helper (Tfh) cells extrafollicular interaction protects from atherosclerosis in mice. Others have shown that Tfh- Germinal Centre (GC) B cell response is proatherogenic. Our preliminary results indicate that MZB cells regulate Tfh differentiation through metabolic reprogramming and we have identified several genes linked to mitochondrial pathways that could play a role. One fo them, the apoptosis inducing factor (Aif1), regulates the complex I of the respiratory chain. Our aim is to investigate the potential role of Aif1 in Tfh functions and its impact on atherosclerosis. Methodology: We generated atherosclerotic mouse models (Ldlr-/-; Rag2-/-) with specific Aif1 deletion in Tfh. Results: Specific Aif1 deletion in Tfh lead to reduced Tfh numbers and subsequent decrease in GC B cells without affecting MZB cell numbers. In females, this lead to a significant increase in atherosclerosis suggesting deletion of Aif1 results in a significant decrease of atheroprotective CuOx LDL and MDA LDL IgM titres, without affecting IgG levels. But in males the effect is opposite; there was a significant reduction of atherogenic CuOxLDL IgG, IgG1 and IgG2c levels without affecting IgM levels. Conclusion: Aif1 regulates Tfh function to produce anti-CuOxLDL and anti-MDA LDL antibodies in a sex dependent manner. In females, Aif1 is necessary for Tfh extrafollicular response and the formation of IgM antibodies. In males, Aif1 is necessary for the Tfh-GC B response and the formation of IgG antibodies. | |
| 15 | Low grade inflammation in Pulmonary Arterial Hypertension is a predictor of mortality and associates with clinical outcomes, partially mediated by obesity |
| Dr Eckart De Bie, Dr Dharshan Giri, Dr Ze Ming Goh, Dr Chris Rhodes, Dr Stefan Gräf, Dr Alexander Rothman, Dr Chris Wallace & Dr Mark Toshner | |
| Pulmonary Arterial Hypertension (PAH) is associated with severe morbidity and mortality. The cause of this remains idiopathic (IPAH) in significant proportion of patients, many of whom have an autoimmune signature (Jones et al. AJRCCM. 2022). Increased levels of C-reactive protein (CRP), a routine biomarker of inflammation, are associated with both survival and clinical parameters in IPAH (Quarck et al. JACC. 2009). However, factors mediating this association have not been identified and large scale studies identifying subgroups are lacking. Using the BHF national cohort study of IPAH/HPAH, time series clustering was employed to identify subgroups of n=246 patients based on CRP trajectory. Additionally, differences between patients with normal levels of CRP (<5mg/l; n=477) and low grade inflammation (>5mg/l; n=276) at diagnosis/the first study visit were assessed. Two clusters were identified; with high CRP and low CRP levels that remained stable over time (Figure 1A). Clusters differed significantly in clinical parameters including increased BMI, higher right atrial pressures, and shorter 6-minute walk distances in the high CRP cluster. Linear models showed that these differences were partially mediated by higher BMI. Patients with low grade inflammation at diagnosis or at the first study visit were at increased risk of mortality (Figure 1B), independent of age at diagnosis, sex, BMI and modified Charlson-Comorbidity Index score, but not independent of haemodynamics. These groups also differed significantly in clinical parameters similarly to the clusters. In conclusion, this study shows that low grade inflammation predicts mortality in IPAH and associates with physiological parameters, partially mediated by obesity. | |
| CARDIOMETABOLIC MEDICINE | |
| 16 | miR-15b-5p, a miRNA programmed by maternal obesity is released in cardiac extracellular vesicles and demonstrates selective uptake. |
| Adriana Córdova-Casanova, Lucas C. Pantaleão, Youguo Niu, Dino A. Giussani, Denise S. Fernandez-Twinn, Susan E. Ozanne | |
| Introduction: Growing evidence supports the hypothesis of developmental programming of health and disease. Environmental and nutritional imbalances during fetal and early post-natal life increase the risk of cardiometabolic diseases. However, the biological mechanisms behind this phenomenon have not been totally elucidated. Here we investigated the specific involvement of miR 15b-5p in the programming of cardiac metabolism by maternal obesity. Methods: We studied the effects of maternal obesity during pregnancy on offspring cardiac miR-15b-5p expression in a murine model. Using a Langendorff preparation, we determined if miR15b-5p was released in extracellular vesicles (EVs) from isolated hearts in response to ischemia-reperfusion in mice. We also studied the uptake of cardiac EVs into different cell lines. Results: miR-15b-5p levels were increased in the hearts of adult mice born to obese dams. We demonstrated that cardiac tissue releases miR-15b-5p during ischemia-reperfusion, and that some of the released miR-15b-5p was contained within EVs. We also showed that the release of miR-15b-5p within EVs was higher in hearts exposed to maternal obesity. Cardiac myoblasts (H9c2) and umbilical endothelial (HUVEC) cell lines readily internalised DiR-labelled cardiac EVs following time exposure, however, preadipocytes (3T3-L1) did not. Conclusions: Our findings suggest that miR-15-5p, a miRNA programmed by maternal obesity is released during ischemia-reperfusion and contained within EVs. These EVs are selectively taken up by cardiac and vascular cell types suggesting possible autocrine/paracrine communication in ischaemic hearts and peripheral vasculature | |
| 17 | Loss of RET-ROS at complex I induces obesity-related diastolic dysfunction in mice that is reversed by aerobic exercise |
| Ana Vujic, Amy Koo, Guillaume Bidault, Andrew M. James, Andreas Dannhorn, Jiro Abe, Jordan J. Lee, Keira Turner, Richard Goodwin, Antonio Vidal Puig, Michael P. Murphy and Thomas Krieg | |
| Heart failure (HF) is one of the most prevalent cardiovascular disease worldwide and a major public health problem. Nearly 50% of patients have heart failure with preserved ejection fraction (HFpEF) and its prevalence is rising. Mitochondrial abnormalities such as alteration of the mitochondrial oxidative function, changes to ATP production and excessive generation of mitochondrial reactive oxygen species (ROS), have been implicated in the pathophysiology of HFpEF, however the exact relationship is unknown. Here, we show that loss of reverse electron transport (RET)-ROS production in vivo, accomplished via a (ND6 G13997A) mtDNA mutation in the ND6 subunit of complex I, results in reduced exercise capacity, and obesity-related HFpEF. Mechanistically, we show that loss of RET-ROS leads to metabolic rewiring towards increased lipid uptake, fatty acid oxidation and altered neutral lipid composition in the heart. Remarkably, endurance exercise prevented body weight gain, normalised lipid composition in the heart and restored diastolic cardiac function. In summary, these data show a crucial role for RET-ROS in regulating exercise capacity and cardiometabolic health in vivo. These findings highlight redox processes and lipid metabolism as potential therapeutic targets for obesity-related HFpEF. | |
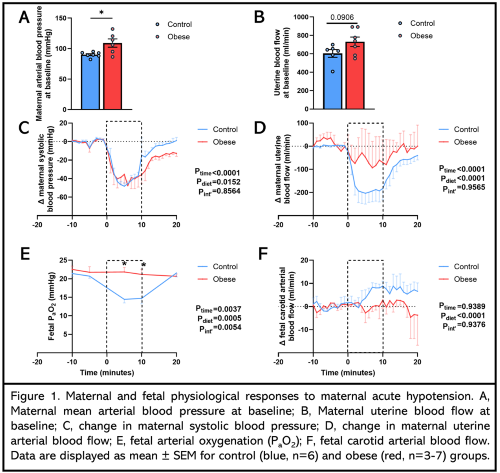
| 18 | Maternal Obesity In Pregnancy Improves Fetal Outcomes to Maternal Hypotension |
| Anna L.K. Cochrane, Rachael C. Crew, Youguo Niu, Sage G. Ford, Clement L. Cahen, Michael P. Murphy, Susan E. Ozanne and Dino A. Giussani | |
|
Introduction: Maternal acute hypotension is a common complication in pregnancy, arising in 75% of cases when spinal anaesthesia is used during elective C-section. Women with high BMI experience higher rates of C-section, however, the impact of obesity on the fetal cardiovascular response to acute hypotension is unknown. Methods: Welsh mountain ewes were fed 60 days pre-pregnancy and throughout gestation with a control (C, recommended concentrates, ad. lib. hay) or obesogenic (OB, ad. lib. concentrates and hay) diet. At 117±2 days gestational age (dGA, term is ca. 147 days), under general anaesthesia, a uterine artery Transonic flow probe was implanted, and catheters and flow probes placed in the fetal carotid and femoral circulations, and the maternal femoral circulation. At 125±2 dGA, sodium nitroprusside (SNP), was infused into the maternal vein (5.6µg/kg/min, for 10 minutes). Data (mean±SEM) were analysed by Student’s t-test or 2-way ANOVA. Results: At baseline, obese ewes were hypertensive, with a trend towards increased uterine blood flow (Figure 1A, B). SNP infusion resulted in a similar fall in maternal systolic blood pressure in both groups (Figure 1C). Control ewes showed a substantial reduction in uterine blood flow during the challenge, which was markedly attenuated in obese ewes (Figure 1D). While control fetuses showed a significant fall in arterial oxygenation and increased carotid blood flow, these effects were absent in fetuses of obese pregnancy (Figure 1E, F). Conclusion: Maternal obesity in pregnancy prevents fetal hypoxia and associated brain-sparing in response to acute maternal hypotension due to improved maintenance of uterine blood flow. |
|
| 19 | The gut microbiome predicts the prospective risk of liver disease |
| Yang Liu, Guillaume Meric, Aki S. Havulinna, Shu Mei Teo, Fredrik Åberg, Matti Ruuskanen, Jon Sanders, Qiyun Zhu, Anupriya Tripathi, Karin Verspoor, Susan Cheng, Mohit Jain, Pekka Jousilahti, Yoshiki Vázquez-Baeza, Rohit Loomba, Leo Lahti, Teemu Niiranen, Veikko Salomaa, Rob Knight, Michael Inouye | |
| While liver diseases are major causes of deaths worldwide, there remains an unmet need for high fidelity early and non-invasive detection. As gut microbiota largely contribute to the reciprocal interactions between the gut and liver and are modifiable through interventions, investigating whether gut metagenomics can be used to stratify liver disease risk may provide new opportunities for prevention. Here, we investigated the potential clinical validity of the gut microbiome for incident liver diseases using shallow metagenomic sequencing of stool samples and machine learning approaches in a large prospective population-based cohort with ~15 years of follow-up through electronic health records. We assessed the predictive capacity of the gut microbiome by itself and in combination with conventional risk factors to improve prediction of alcoholic liver disease and a broader spectrum of liver diseases. We demonstrated that the gut microbiome-based models exhibited comparable prediction performance to the conventional models for liver diseases. The models integrating the gut microbiome and conventional risk factors using machine learning markedly improved the prediction performance to average area under the curve (auc) of 0.834 for general liver disease and 0.956 for alcoholic liver disease with improved risk stratification as compared to conventional risk factors alone. Furthermore, the models combining the gut microbiome and conventional risk factors outperformed clinical non-invasive tests Fatty Liver Index and BARD score for prediction of incident liver diseases. This study indicates that the combination of conventional risk factors with gut microbiota may have potential clinical utility in early risk stratification for liver disease. | |
| 20 | Early Origins of Cardiac Dysfunction in an Embryonic Chicken Model of Obstructive Sleep Apnoea During Development |
|
Youguo Niu, Sara Perelmuter, Natasha I. Cavell, Rachael C. Crew, Jack P. Barry, Sage G. Ford, Anna L. Cochrane, German A. Arenas, Lucas C. Pantaleao, Bernardo J. Krause, Susan E. Ozanne & Dino A. Giussani |
|
| Background: Obstructive sleep apnoea (OSA) induces intermittent hypoxia (IH). In turn, pregnancy and obesity promote OSA (Senaratna. Sleep Med Rev 34: 70, 2017), and maternal obesity during pregnancy is increasing. However, the effects of IH during pregnancy on offspring cardiovascular health are unknown. We tested the hypothesis that IH triggers developmental origins of cardiac dysfunction using the chicken embryo. Methods: Fertilised Bovans Brown eggs (n=52) were incubated under normoxia (O2: 21%) or IH (O2: 21%-13%, 30 cycles / hour; BioSpherix OxyCycler) to mimic OSA in pregnancy from day 1 of the 21-day incubation. On day 19, following cervical transection and biometry, cardiac function was determined via Langendorff. In a separate cohort, cardiac molecular analysis was performed (RT-qPCR). Data were analysed by Student’s t test or RM ANOVA. P<0.05 was considered significant. Results: IH embryos showed asymmetric growth restriction (Fig. 1A & B), cardiac dysfunction (Fig. 1C & D) and impaired calcium signalling via reduced phospholamban (PLN; Fig. 1E) compared to controls. They also exhibited cardiac sympathetic dominance (Fig. 1F), down-regulated muscarinic receptor signalling (Fig. 1G), impaired cardiac recovery to an ischaemia/reperfusion (IR) challenge (Fig. 1H) and a decrease in the expression of the cardioprotective gene, protein kinase C epsilon (PKCɛ; Fig1I). Other pathways triggered by IH included increased markers of inflammation (Fig. 1J). Cardiac dysfunction and risk of IR injury was greater in male than in female IH embryos (data not shown). Conclusions: IH during fetal development, as in pregnancy affected by OSA, triggers an embryonic origin of heart disease. | |
| TRANSLATION | |
| 21 | Correcting Common OCT Artifacts Enhances Plaque Classification and Identification of Higher-Risk Plaque Features |
| Benn Jessney, Xu Chen, Sophie Gu, Adam Brown, Daniel Obaid, Charis Costopoulos, Martin Goddard, Hector Garcia-Garcia, Yoshinobu Onuma, Patrick Serruys, Stephen P Hoole, Michael Mahmoudi, Michael Roberts, Martin Bennett | |
|
Background: Optical coherence tomography (OCT) is used widely to guide stent placement, identify higher-risk plaques, and assess mechanisms of drug efficacy. However, a range of common artifacts can prevent accurate plaque classification and measurements, and limit usable frames in research studies. Aims: To determine whether pre-processing OCT images corrects artifacts and improves plaque classification. Methods: We examined both ex-vivo and clinical trial OCT pullbacks for artifacts that prevented accurate tissue identification and/or plaque measurements. We developed Fourier transform-based software that reconstructed images free of common OCT artifacts, and compared corrected and uncorrected images. Results: 48% of OCT frames contained image artifacts, with 62% of artifacts over or within lesions, preventing accurate measurement in 12% frames. Pre-processing corrected >70% of all artifacts, including thrombus, macrophage shadows, inadequate flushing, and gas bubbles. True tissue reconstruction was achieved in 63% frames that would otherwise prevent accurate clinical measurements. Artifact correction was non-destructive and retained anatomical lumen and plaque parameters. Correction improved accuracy of plaque classification compared against histology and retained accurate assessment of higher-risk features. Correction also changed plaque classification and prevented artifact-related measurement errors in a clinical study, and reduced unmeasurable frames to <5% ex-vivo and ~1% in-vivo. Conclusions: Fourier transform-based pre-processing corrects a wide range of common OCT artifacts, improving identification of higher-risk features and plaque classification, and allowing more of the whole dataset to be used for clinical decision-making and in research. Pre-processing can augment OCT image analysis systems both for stent optimisation and in natural history or drug studies. |
|
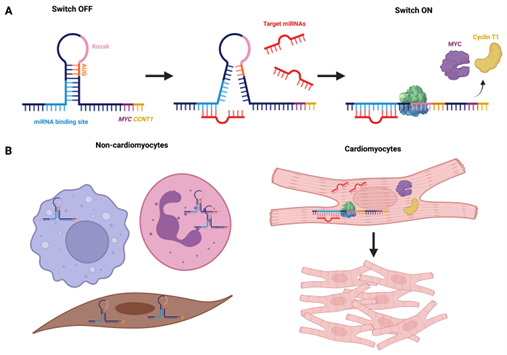
| 22 | Development of a cardiomyocyte-specific mRNA nanodevice |
| Elsa Lawrence, Prof. Sanjay Sinha and Dr. Catherine Wilson | |
|
Cardiomyocyte (CM) death, for example following myocardial infarction (MI), can have life-threatening or disabling consequences. The inability of adult heart muscle to regenerate therefore poses a major fundamental challenge in treating heart disease. Reperfusion therapies restore blood flow and limit damage, but do not allow regeneration of lost muscle tissue and function. Considering these limitations, we are developing a novel method for stimulating endogenous cardiac regeneration. The Wilson Lab has recently discovered that transient co-expression of Myc and Cyclin T1 can elicit cellular proliferation of adult mammalian CMs in mouse models in vivo. We are translating these findings using a modified synthetic messenger RNA (modRNA) for combined Myc-Cyclin T1 delivery. Early results indicate Myc-Cyclin T1 modRNA therapy improves left ventricular function and increases CM number when administered immediately following MI in adult mice. I am improving this modRNA therapeutic by developing a tissue-specific mRNA switch system. The switch contains a recognition sequence half concealed by a hairpin that requires CM-specific microRNA (miRNA) binding for protein translation to occur. Thus, in non-CMs, translation will be significantly limited. I have shown that translation of reporter mRNA is impeded with the addition of a hairpin structure in the 5’UTR and that cardiomyocyte-specific 499a-5p reporter hairpin mRNA expresses in cardiomyocytes in vitro. Translation of miR-499a-5p hairpin mRNA can be increased using miRNA mimics in rabbit reticulocytes. The hairpins will be administered into mice and assessed. This tissue-specific mRNA switch system could potentially be applied to any tissue-specific disease with targetable miRNAs. |
|
| 23 | An adjunct therapy to mechanical thrombectomy – locally administered acidified malonate protects against stroke ischaemia/reperfusion injury |
| Jordan J Lee, Hiran A Prag, Karthik Chary, Jiro Abe, Shinpei Uno, Annabel Sorby-Adams, Chak Shun Yu, Olga Sauchanka, Amin Mottahedin, Joshua Kaggie, Ferdia Gallagher, Michael P Murphy, Thomas Krieg | |
| Background and aims Mechanical thrombectomy provides the opportunity for targeted drug delivery into ischaemic tissue upon reperfusion; however, equivalent pre-clinical animal models are lacking. Malonate, a competitive inhibitor of succinate dehydrogenase, preferentially enters ischaemic tissue on reperfusion via monocarboxylate transporter 1 (MCT), and potentially MCT2 and MCT4 due to the low tissue pH and ameliorates ischaemia/reperfusion injury (IRI). We aimed to develop a method for the local administration of acidified malonate and determine its efficacy as an adjunct therapy in a pre-clinical model of stroke. Methods C57BL/6J mice were treated with saline or malonate at physiological or low pH (6.0) either intravenously or intra-arterially via the thrombectomy catheter. Metabolites were measured by liquid chromatography-tandem mass spectrometry. For transient middle cerebral artery occlusion (tMCAO), mice were subjected to 30 mins ischaemia followed by either 2 or 24 hours reperfusion. Brain infarct size was assessed by TTC-staining or MRI. mtDNA amplification, complex I activity, and lipid peroxidation (MDA) assays were used as oxidative damage markers. Results Intravenous malonate treatment significantly reduced infarct size at both 2 and 24 hours post-stroke compared to saline controls. Administration of acidified malonate locally to drive its uptake via MCT1 significantly enhanced brain uptake, consequently leading to reduced infarct size at lower administered doses. In addition, local acidified malonate administration significantly reduced the extent of oxidative damage, mean apparent diffusion coefficient and neurological score were also improved. Conclusions Malonate is neuroprotective against stroke IRI, and is an attractive candidate as an adjunct therapy for mechanical thrombectomy. | |
| 24 | Integrating immune cells to 3D cardiac microtissues for in vitro cardiac safety assessment of novel therapeutics with immune pharmacodynamics |
| Jose Vicencio, Carol De Santis, Semiramis Popova, Lucy Flint | |
| Novel therapeutic modalities with immune pharmacodynamics (e.g immune checkpoint inhibitors, ICI) have clinically exhibited myocarditis and other immune-mediated adverse reactions. To evolve preclinical cardiac safety assessment towards incorporating these liabilities, we have introduced immune cells to 3D cardiac microtissues (3D-CMT) in vitro. Our work shows that human peripheral blood mononuclear cells (PBMC) infiltrate human 3D-CMTs in response to pro-inflammatory stimulation and to multiple therapeutic modalities including ICI and adeno-associated virus (AAV). Establishing these readouts additionally revealed that cardiac immunocompetent co-cultures require a degree of donor-compatibility. To achieve this, our 3D-CMT (composed of cardiomyocytes, endothelial cells and fibroblasts from different donors) were mono-allelically matched with PBMCs in HLA-A/B/C. To phenotype cardiac infiltrating immune populations, imaging mass cytometry (IMC) revealed increased CD8+ T cell infiltration in response to the widely used ICI atezolizumab. Our data supports further mechanistic discovery of markers linked to therapy-induced myocarditis and contributes to strengthen preclinical cardiac safety assessment in face of novel modalities. | |
| 25 | The digital 1-Minute Walk Test: a new patient-centred endpoint for cardiopulmonary clinical trials |
| Dr Joseph Newman & Dr Mark Toshner | |
| Background: The 6-Minute Walk Test (6MWT) is well established and widely used in cardiorespiratory disease, especially in pulmonary arterial hypertension (PAH) trials. The 6MWT could be digitalised and shortened to develop a patient-centred endpoint suitable for community deployment. Objectives: To develop a community-based functional capacity endpoint evolved from the gold-standard 6WMT. Methods: Patient views on digital clinical trials were explored by an online national survey (n=112), initiating an endpoint co-development process. A prospective study of timed walk distances derived from shorter durations was conducted in two diseases, PAH and interstitial lung disease (ILD)(n=193), examining walk periods of 1/2/3/4/5/6 minutes. The shortest period was taken forward for digital configuration and validation. The 1MWT captured by a smartphone application and paired wearable device was undertaken and compared against the 6MWT. Results: Surveyed patients viewed digital and decentralised clinical trial methodology favourably. The distance walked in the first minute was highly correlated with the total 6MWT distance (6MWD) in both disease cohorts (r=0.96) with equivalent 3-year mortality prediction; AUC in PAH 1MWD was 0.769(p<0.001) and 6MWD was 0.799(p<0.001), and AUC in ILD 1MWD was 0.696(p<0.001) and 6MWD 0.689(P<0.001). The digital 1MWD correlated with both the ground truth distance (Figure1A;r=0.92,p<0.001) and the gold-standard 6MWD (r=0.89,p<0.001) for n=24. Patients expressed a preference for shorter walk tests and remote testing via smart technology(Figure1B). >200 independent and remote d1MWTs have been completed with good longitudinal adherence, repeatability and usability scores. Conclusions: The digital 1MWT co-developed with patients shows technical accuracy, repeatability, construct validity and patient acceptability. | |
- giving a Poster Preview in the 10:50 session