This page collects research news and articles that we have published into one place. The purpose of this page is to highlight some ongoing projects that will benefit patients in future and to give more visibility to BHF-funded Group Leaders, Research Associates and Postgraduate Students. Please contact webmaster@cardiovascular.cam.ac.uk if you would like your research to be featured here.
The Louis & Arthur Lucian Award for Research in Circulatory Diseases
Many congratulations to Ziad Mallat a BHF Professor of Cardiovascular Medicine at the Victor Phillip Dahdaleh Heart and Lung Research Institute. Ziad has been awarded the Louis and Artur Lucian Award for Research in Circulatory Diseases due to his major contributions to cardiovascular immunology.
The Louis and Artur Lucian Award was established through a bequest to McGill University under the will of the late Olga Leibovici to honour the donor's two brothers and was conferred for the first time in 1978. The Award is designed to honour outstanding research in the field of circulatory diseases by a scientific investigator or group of investigators whose contribution to knowledge in this field is deemed worthy of special recognition.
Modernized anti-arrhythmic drug classification gains global recognition
Professors Ming Lei and Derek Terrar (Oxford University), Christopher Huang (University of Cambridge), and Lin Wu (Beijing University Hospital) have further developed their 2018 Oxford modernized antiarrhythmic drug (AAD) classification into a practical, more clinically useful, framework in a newly published (2025) article in Heart Rhythm, the official journal of the Heart Rhythm Society (USA) (Heart Rhythm. 2025 Apr 3:S1547-5271). This evolution in AAD classification represents a major step to advance harmonization of therapeutic strategies and drug development across global healthcare systems. The Modernized Oxford AAD classification is poised to become the standard reference in clinical cardiology for managing cardiac arrhythmias.
Cardiac arrhythmias pose a serious global health challenge. The commonest sustained arrhythmia atrial fibrillation (AF) affects 1–2% of the worldwide population. Antiarrhythmic drugs (AADs) remain central to their pharmacotherapeutic management.
The major approach to advancing cardiac arrhythmia management has been recognized in international clinical guidelines for AAD management, in the latest Clinical Consensus Statement from the Task Force of the European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC). A panel of internationally recognized cardiology experts highlighted and adopted the modernized 2018 Oxford classification as an essential improvement on the classic VW system.
The modernized Oxford AAD classification has been as the most comprehensive and clinically relevant revision of the VW system to date. The updated scheme systematizes a broad range of drug classes and subclasses, placing them within two overarching categories: regulatory-approved, clinically used AADs (Classes 0, I–IV, and VII) and investigational new drugs under development (INDs; Classes V and VI). The former includes existing off-label formulations (e.g ivabradine and ranolazine in Classes 0 and Id respectively).
References:
Lei M, Wu L, Terrar DA, Huang CL. Modernized Classification of Cardiac Antiarrhythmic Drugs. Circulation. 2018 Oct 23;138(17):1879-1896. doi: 10.1161/CIRCULATIONAHA.118.035455.
Lei M, Wu L, Terrar DA, Huang CL. The modernized classification of cardiac antiarrhythmic drugs: Its application to clinical practice. Heart Rhythm. 2025 Apr 3:S1547-5271(25)02300-8. doi: 10.1016/j.hrthm.2025.03.1997.
Merino JL et al. Practical Compendium of Antiarrhythmic Drugs: A Clinical Consensus Statement of the European Heart Rhythm Association of the ESC . EP Europace, euaf076, https://doi.org/10.1093/europace/euaf076
Low-dose interleukin-2 induces clonal expansion of BACH2-repressed effector regulatory T cells following acute coronary syndrome
Targeting inflammation in atherosclerotic cardiovascular disease remains a major unmet need. Low-dose interleukin-2 (IL-2LD) selectively increases regulatory T (Treg) cell numbers in patients with coronary artery disease.
Using single-cell transcriptomics and T cell receptor analyses, Tian Zhao, Ziad Mallat and researchers from Cambridge University show that IL-2LD clonally expands effector Treg cells in patients with acute coronary syndromes. The clonally expanded Treg cells upregulate key immunosuppressive and metabolic pathways and show an increased number of predicted ligand–receptor interactions. These Treg cells also display similar predicted antigen specificities, which cluster with published sequences specific to atherosclerotic cardiovascular disease. By tracking the T cell receptors of single cells over time, they identify an inflammatory polarization of the T cell compartment after myocardial infarction, which is restrained by IL-2LD. BACH2 is identified as a repressor of the Treg effector program. However, BACH2-mediated regulation is bypassed with IL-2LD. Overall, these results lend insight into the IL-2-driven clonal expansion program in human Treg cells, with important therapeutic implications for patients with cardiovascular and other immune-mediated diseases.
Reference
Case, A.G., O’Brien, J.W., Lu, Y. et al. Low-dose interleukin-2 induces clonal expansion of BACH2-repressed effector regulatory T cells following acute coronary syndrome. Nat Cardiovasc Res 4, 727–739 (2025). https://www.nature.com/articles/s44161-025-00652-y
Targeting gut microbiota to regulate the adaptive immune response in atherosclerosis
Accumulating evidence indicates the important regulatory role of the adaptive immune system in atherosclerosis during all stages of the disease. The gut microbiome has also become a key regulator of atherosclerosis and immunomodulation.
This review carried out by Dr Meritxell Nus, Dr Despoina Giakomidi and Ayoola Ishola explores potential strategies to therapeutically target the microbiome, including the use of prebiotics and vaccinations, which could influence the adaptive immune response and consequently plaque composition and development.
BHF funded PhD Student Anna Cochrane selected to attend the Stockholm International Youth Science Seminar (SIYSS)
The history of SIYSS dates back to 1976 when the first seminar was organized by the Swedish Federation of Young Scientists, with inspiration from Society for Science & the Public in USA. Turning out to be a great success, the SIYSS program has continued, combining the encounter with Swedish science and the Nobel Prize Awarding Ceremonies.The participants are selected in different ways; some are winners of national science fairs, others represent organizations for young scientists or are selected by merit at their home universities. Anna's work.
Early intermittent hyperlipidaemia alters tissue macrophages to fuel atherosclerosis
Hyperlipidaemia is a major risk factor of atherosclerotic cardiovascular disease (ASCVD). Risk of cardiovascular events depends on cumulative lifetime exposure to low-density lipoprotein cholesterol (LDL-C) and, independently, on the time course of exposure to LDL-C, with early exposure being associated with a higher risk1. Furthermore, LDL-C fluctuations are associated with ASCVD outcomes2-4. However, the precise mechanisms behind this increased ASCVD risk are not understood. Here, we make the unexpected observation that early intermittent feeding of mice with a high-cholesterol Western-type diet (WD) accelerates atherosclerosis compared with late continuous exposure to WD, despite similar cumulative circulating LDL-C levels. We find that early intermittent hyperlipidaemia alters the number and homeostatic phenotype of resident-like arterial macrophages. Macrophage genes with altered expression are enriched for genes linked to human ASCVD in genome-wide association studies. We show that LYVE1+ resident macrophages are atheroprotective, and identify new biological pathways, related to actin filament organisation, whose alteration accelerates atherosclerosis. Using the Young Finns Study, we show that exposure to cholesterol early in life is significantly associated with the incidence and size of carotid atherosclerotic plaques in mid-adulthood. In summary, our results identify early intermittent exposure to cholesterol as a strong determinant of accelerated atherosclerosis, highlighting the importance of optimal control of hyperlipidaemia early in life, and providing insight into the underlying biological mechanisms. This knowledge will be essential to designing effective therapeutic strategies to combat atherosclerotic cardiovascular disease.
Minoru Takaoka, Xiaohui Zhao, Hwee Ying Lim, Costan G. Magnussen, Owen Ang, Nadine Suffee, Patricia R. Schrank, Wei Siong Ong, Dimitrios Tsiantoulas, Felix Sommer, Sarajo K. Mohanta, James Harrison, Yaxing Meng, Ludivine Laurans, Feitong Wu, Yuning Lu, Leanne Masters, Stephen A. Newland, Laura Denti, Mingyang Hong, Mouna Chajadine, Markus Juonala, Juhani S. Koskinen, Mika Kähönen, Katja Pahkala, Suvi P. Rovio, Juha Mykkänen, Russell Thomson, Tsuneyasu Kaisho, Andreas J. R. Habenicht, Marc Clement, Alain Tedgui, Hafid Ait-Oufella, Tian X. Zhao, Meritxell Nus, Christiana Ruhrberg, Soraya Taleb, Jesse W. Williams, Olli T. Raitakari, Véronique Angeli & Ziad Mallat
COVID-19 related myocardial injury is associated with immune dysregulation in symptomatic patients with cardiac MRI abnormalities
While acute cardiovascular complications of COVID-19 are well-described, less is known about longer-term cardiac sequelae. For many individuals with cardiac signs or symptoms arising after COVID-19 infection, the aetiology remains unclear. We examined immune profiles associated with magnetic resonance imaging (MRI) abnormalities in patients with unexplained cardiac injury after COVID-19.
Andrej Ćorović 1, Xiaohui Zhao 1, Yuan Huang 1, Stephen Newland 1, Deepa Gopalan 2, James Harrison 1, Despina Giakomidi 1, Shanna Chen 1, Natalia S Yarkoni 3, Christopher Wall 1, Marta Peverelli 1, Rouchelle Sriranjan 1, Arianna Gallo 1, Martin J Graves 4, Andrew Sage 1, Paul A Lyons 5 6, Nyarie Sithole 7, Martin R Bennett 1, James H F Rudd 1, Ziad Mallat 1, Tian X Zhao 1, Meritxell Nus 1, Jason M Tarkin 1
Study explores the strategy behind one of the world's most successful labs
A recent study published in Nature by Cambridge researchers Antonio Vidal-Puig from the Institute of Metabolic Sciences, Chander Velu from the Institute for Manufacturing, and Luka Gebel from King’s College London explores the strategic management practices that contribute to the success of research-intensive institutions. Their findings highlight the Laboratory of Molecular Biology (LMB) at Cambridge as a model of excellence in scientific innovation.
The collaborative study, which brings together insights from the fields of biosciences, engineering, and business, underscores the significance of effective management in driving scientific breakthroughs. By examining LMB’s approach, the study identifies three key principles that are instrumental in its achievements: a dynamic organisational culture, strategic incentive structures, and balanced management oversight.
This study exemplifies the importance of interdisciplinary collaboration, drawing on the expertise of the Institute of Metabolic Sciences, the VPD Heart and Lung Research Institute, the Institute for Manufacturing, and the Judge Business School. By integrating knowledge from diverse fields, research-intensive institutions can create environments conducive to innovation and scientific breakthroughs.
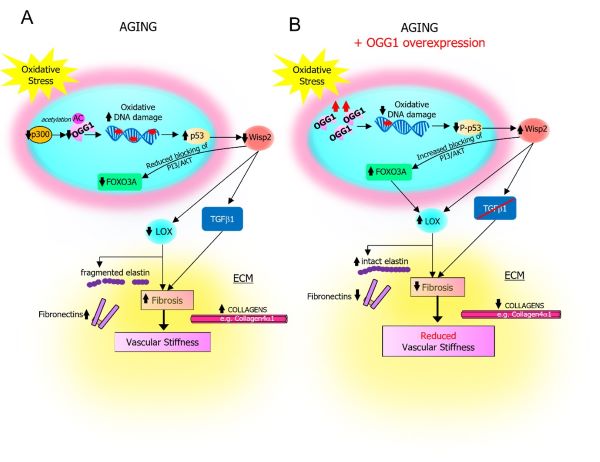
Oxidative DNA damage promotes vascular ageing associated with changes in extracellular matrix-regulating proteins
Vascular ageing is characterised by oxidative DNA damage, vessel stiffening and increased deposition of extracellular matrix (ECM) proteins; however, mechanisms linking these features are largely unknown. In a study published in Cardiovascular Research on 8th May 2024, researchers at the VPD-HLRI showed that aged arteries from mice and humans have higher oxidative DNA damage, with reduced expression and activity of proteins that regulate oxidative repair, and reduced expression of multiple ECM regulators. The study, led by Dr Kirsty Foote, demonstrated that enhancing the base excision repair enzyme OGG1 in vascular smooth muscle cells protected arteries from age, and restored the expression of ECM regulators that reduce age-associated fibrosis (including LOX and WISP2/CCN5). This work uncovers a new link coupling defects in oxidative DNA repair with changes in ECM proteins that lead to vascular stiffness, and highlights the importance of maintaining oxidative DNA repair as a potential therapeutic target in slowing vascular ageing.
This effort was a collaboration with laboratories at Kings College London, the Wellcome Trust Sanger Institute, the University of Manchester and colleagues at the University of Cambridge.
Schematic illustrating changes in Ogg1, Foxo3a, Wisp2, Lox, Tgfb and ECM proteins in vascular ageing and after Ogg1 overexpression.
Kirsty Foote, Marieke Rienks, Lukas Schmidt, Konstantinos Theofilatos, Yasmin, Matiss Ozols, Alexander Eckersley, Aarti Shah, Nichola Figg, Alison Finigan, Kevin O’Shaughnessy, Ian Wilkinson, Manuel Mayr, Martin Bennett.
DOI: 10.1093/cvr/cvae091
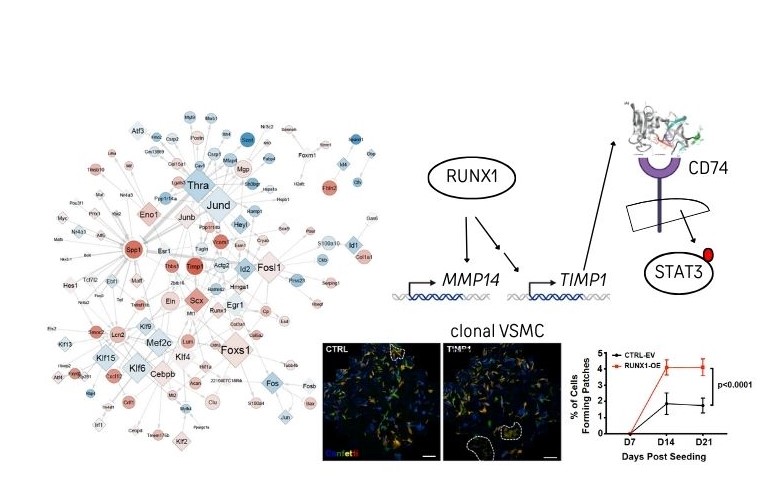
Network-based prioritisation and validation of regulators of vascular smooth muscle cell proliferation in disease
Vascular smooth muscle cells (VSMCs) are important for blood vessel function but also generate the majority of cells in atherosclerotic lesions, which cause heart attack and stroke. In a study published in Nature Cardiovascular Research on 6th June 2024, Dr Helle Jørgensen’s team at the VPD-HLRI used computational analysis to demonstrate that VSMC proliferation is accompanied by widespread regulatory changes that could be exploited to reduce vascular disease susceptibility and for disease prevention strategies. Experimental network verification highlighted the pioneer transcription factor RUNX1 as a novel VSMC regulator. The study, which was driven by Dr Jordi Lambert and Dr Sebnem Oc, also demonstrated that TIMP1 acts as a cytokine to promote VSMC proliferation through signalling via CD74 andSTAT3. Both RUNX1 and the TIMP1-CD74-STAT3 axis are expressed in human VSMCs, suggesting clinical relevance and potential as vascular disease targets
This effort was a collaboration with laboratories at Imperial College/MRC Laboratory of Medical Sciences, University of Leicester, Technical University of Munich and colleagues at the University of Cambridge.
DOI: 10.1038/s44161-024-00474-4.
Bone morphogenetic protein signalling in pulmonary arterial hypertension: revisiting the BMPRII connection
Wei Li and colleagues have published a new mini-review on BMP Signalling in PAH. Pulmonary arterial hypertension (PAH) is a rare and life-threatening vascular disorder, characterised by abnormal remodelling of the pulmonary vessels and elevated pulmonary artery pressure, leading to right ventricular hypertrophy and right-sided heart failure. The importance of bone morphogenetic protein (BMP) signalling in the pathogenesis of PAH is demonstrated by human genetic studies. Many PAH risk genes are involved in the BMP signalling pathway and are highly expressed or preferentially act on vascular endothelial cells. Endothelial dysfunction is recognised as an initial trigger for PAH, and endothelial BMP signalling plays a crucial role in the maintenance of endothelial integrity. BMPR2 is the most prevalent PAH gene, found in over 80% of heritable cases. As BMPRII protein is the major type II receptor for a large family of BMP ligands and expressed ubiquitously in many tissues, dysregulated BMP signalling in other cells may also contribute to PAH pathobiology. Sotatercept, which contains the extracellular domain of another transforming growth factor-β family type II receptor ActRIIA fused to immunoglobin Fc domain, was recently approved by the FDA as a treatment for PAH. Neither its target cells nor its mechanism of action is fully understood. This review will revisit BMPRII function and its extracellular regulation, summarise how dysregulated BMP signalling in endothelial cells and smooth muscle cells may contribute to PAH pathogenesis, and discuss how novel therapeutics targeting the extracellular regulation of BMP signalling, such as BMP9 and Sotatercept, can be related to restoring BMPRII function.
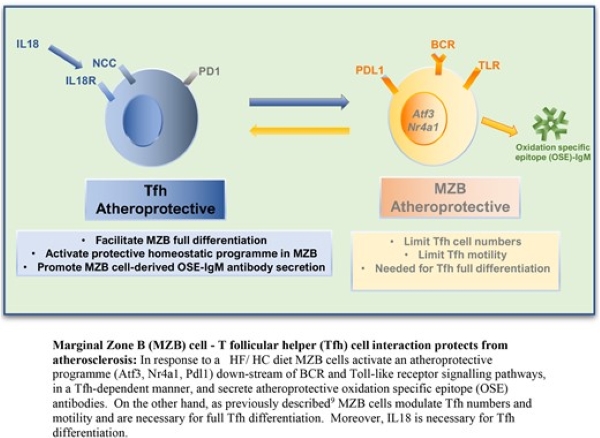
Marginal zone B cells produce ‘natural’ atheroprotective IgM antibodies in a T cell–dependent manner
Meritxell Nus and colleagues* studied how the adaptive immune response plays an important role in atherosclerosis. In response to a high-fat/high-cholesterol (HF/HC) diet, marginal zone B (MZB) cells activate an atheroprotective programme by regulating the differentiation and accumulation of ‘poorly differentiated’ T follicular helper (Tfh) cells. On the other hand, Tfh cells activate the germinal centre response, which promotes atherosclerosis through the production of class-switched high-affinity antibodies. They therefore investigated the direct role of Tfh cells and the role of IL18 in Tfh differentiation in atherosclerosis.
Their findings revealed a previously unsuspected role of MZB cells in regulating atheroprotective ‘natural’ IgM antibody production in a Tfh-dependent manner, which could have important pathophysiological and therapeutic implications.
*James Harrison, Stephen A Newland, Wei Jiang, Despoina Giakomidi, Xiaohui Zhao, Marc Clement, Leanne Masters, Andrej Corovic, Xian Zhang, Fabrizio Drago, Marcella Ma, Maria Ozsvar Kozma, Froher Yasin, Yuta Saady, Hema Kothari, Tian X Zhao, Guo-Ping Shi, Coleen A McNamara, Christoph J Binder, Andrew P Sage, Jason M Tarkin, Ziad Mallat,
A novel human iPSC model of COL4A1/A2 small vessel disease unveils a key pathogenic role of matrix metalloproteinases
Alessandra Granata (Clinical Neurosciences) and colleagues have grown small blood vessel-like models in the lab and used them to show how damage to the scaffolding that supports these vessels can cause them to leak, leading to conditions such as vascular dementia and stroke. Cerebral small vessel disease (SVD) affects the small vessels in the brain and is a leading cause of stroke and dementia. Emerging evidence supports a role of the extracellular matrix (ECM), at the interface between blood and brain, in the progression of SVD pathology, but this remains poorly characterized. To address ECM role in SVD, we developed a co-culture model of mural and endothelial cells using human induced pluripotent stem cells from patients with COL4A1/A2 SVD-related mutations. This model revealed that these mutations induce apoptosis, migration defects, ECM remodeling, and transcriptome changes in mural cells. Importantly, these mural cell defects exert a detrimental effect on endothelial cell tight junctions through paracrine actions. COL4A1/A2 models also express high levels of matrix metalloproteinases (MMPs), and inhibiting MMP activity partially rescues the ECM abnormalities and mural cell phenotypic changes. These data provide a basis for targeting MMP as a therapeutic opportunity in SVD. Coverage included: Times, Independent, Evening Standard and ABC (Spanish) newspapers.
A rare selenoprotein deficiency disorder predisposes to aortic aneurysm formation
Aortic aneurysms, which may dissect or rupture acutely and be fatal, can have a heritable basis. In a rare example, local patients with a deficiency of selenocysteine-containing proteins, due to defects in a gene (SECISBP2) which controls their production, were found to develop progressive, aneurysmal dilatation of the ascending aorta at a young age. In research published in Nature Communications, Erik Schoenmakers (Chatterjee group, Wellcome-MRC Institute of Metabolic Science), Helle Jørgensen and Martin Bennett (Cardiorespiratory Medicine), working with clinical (CUH, RPH) and basic (Mike Murphy, MRC MBU) colleagues, have established a causal relationship, showing that aortas from patients or mice (with vascular smooth muscle cell-specific) selenoprotein deficiency exhibit oxidative stress-mediated cell death, including via ferroptosis, leading to aortic degeneration. Mitochondria-targeted antioxidant exposure prevents oxidative damage in patient’s cells and aortopathy in a zebrafish model, raising the possibility of such therapies in this new syndromic cause of aortic aneurysm. Published 02 December 2023.
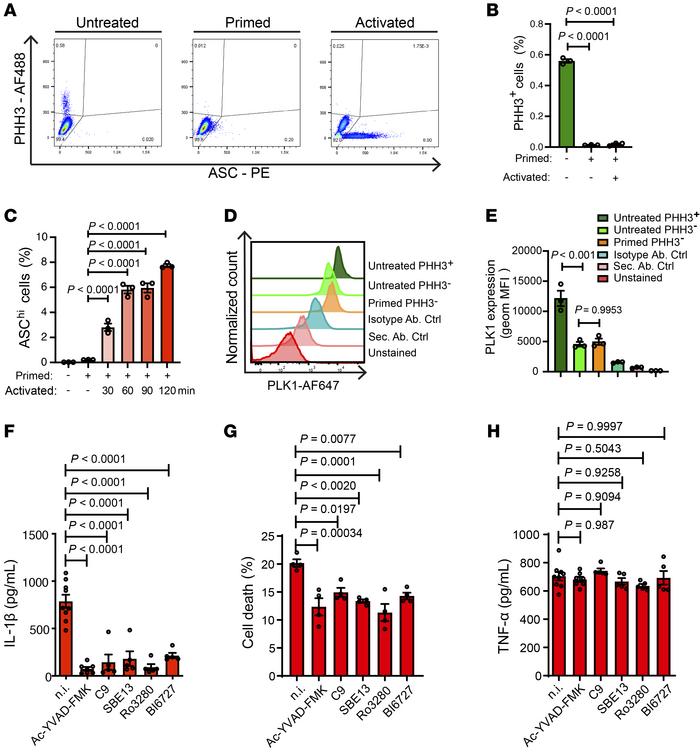
PLK1 inhibition dampens NLRP3 inflammasome–elicited response in inflammatory disease models
A cancer drug currently in the final stages of clinical trials could offer hope for the treatment of a wide range of inflammatory diseases, including gout, heart failure, cardiomyopathy, and atrial fibrillation. "We believe [our findings] could be important in preventing a number of common diseases that can cause pain and disability and in some cases can lead to life-threatening complications" - Xuan Li
In a study published on 1 November 2023 in the Journal of Clinical Investigation, the researchers have identified a molecule that plays a key role in triggering inflammation in response to materials in the body seen as potentially harmful.
We are born with a defence system known as innate immunity, which acts as the first line of defence against harmful materials in the body. Some of these materials will come from outside, such as bacterial or viral infections, while others can be produced within the body.
Innate immunity triggers an inflammatory response, which aims to attack and destroy the perceived threat. But sometimes, this response can become overactive and can itself cause harm to the body.
One such example of this is gout, which occurs when urate crystals build up in joints, causing excessive inflammation, leading to intense pain. Another example is heart attack, where dead cell build up in the damaged heart – the body sees itself as being under attack and an overly-aggressive immune system fights back, causing collateral damage to the heart.
Several of these conditions are characterised by overactivation of a component of the innate immune response known as an inflammasome – specifically, the inflammasome NLRP3. Scientists at the Victor Phillip Dahdaleh Heart and Lung Research Institute at Cambridge have found a molecule that helps NLRP3 respond.
This molecule is known as PLK1. It is involved in a number of processes within the body, including helping organise tiny components of our cells known as microtubules cytoskeletons. These behave like train tracks inside of the cell, allowing important materials to be transported from one part of the cell to another.
Link to article in the Sunday Mirror
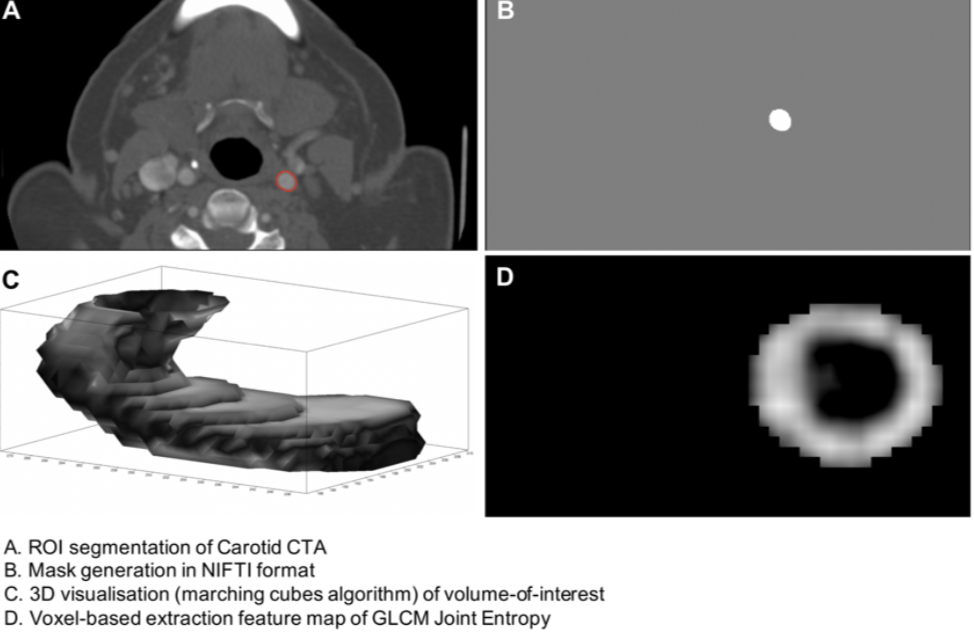
Radiomics in stroke medicine: assessing robustness and feasibility for stroke prevention
A cross-discipline group of researchers, led by MB PhD student Elizabeth Le and PI James Rudd, have published in Nature Scientific Reports the first attempt to determine whether radiomics might be useful for predicting stroke in patients with carotid artery disease. Radiomics refers to the harnessing of information 'hidden' within medical images for diagnostic and prognostic purposes, and is being increasingly used in oncology.
CRE Funded projects
A new online series of short personal interviews with Cambridge Stem Cell Institute researchers exploring how they are adapting their research to investigate Covid-19.
Professor Toni Vidal-Puig, Reflecting on the Covid-19 pandemic and its impact on the research landscape
Professor Antonio Vidal-Puig is Professor of Molecular Nutrition and Metabolism at the University of Cambridge. Reflecting on the Covid-19 pandemic and its impact on the research landscape. Toni tells in this interview with the BHF how the BHF CRE have supported the research, about fundraising for PPE, testing the strength of global networks, and what we can learn from the crisis
BHF CRE Funded Covid projects
Cambridge Investigation of Cardiac Complications of Severe COVID-19 (CICERO-19) - Dr Sanjay Sinha, Prof. Anthony Davenport (Clinical Pharmacology), Prof. Paul Lehner (Medicine), Dr Nick Matheson (Medicine).
Molecular mechanism underlying late-stage pathology in COVID19 – platelets and megakaryocytes- Cedric Ghevaert, Amie Waller
For any information or queries related to Covid-19 topics regarding Cambridge Infectious Diseases IRC, Cambridge Immunology Network SRN, and the Cambridge Cardiovascular IRC, please email covid-response@cam.ac.uk and include CINCID in the subject line.
The Naked Scientists: Covid-19
150 scientists from new institute join Cambridge fight against COVID-19
Past Articles
- Dysregulation of macrophage PEPD in obesity determines adipose tissue fibro-inflammation and insulin resistance (April 2022)
- Pericoronary adipose tissue density is associated with inflammatory disease activity in Takayasu arteritis (September 2021)
- SIRT6 Protects Smooth Muscle Cells from Senescence and Reduces Atherosclerosis Mandy Grootaert, Alison Finigan, Nichola Figg, Anna Katarzyna Uryga, and Martin Bennett
- Understanding the management of heart failure with preserved ejection fraction: a qualitative multiperspective study. Dr Christi Deaton, Florence Nightingale Foundation Clinical Professor of Nursing, Department of Public Health & Primary Care. University of Cambridge School of Clinical Medicine
- Glycoprotein-VI: a stroke of luck in understanding blood clot formation Dr Isuru Induruwa is a BHF-funded clinical PhD Fellow (Dept Clinical Neurosciences) and an ST5 in Stroke Medicine (CUH).
- Next-generation materials for heart valve replacement Prof Geoff Moggridge is a Professor in Chemical Engineering Science (Dept Chemical Engineering & Biotechnology).
- Engineered platelets could meet demand and mend a broken heart Dr Cedric Ghevaert is a Senior Lecturer in Transfusion Medicine (Dept Haematology).
- Functional genomics: Shedding light on cardiovascular disease Dr Mattia Frontini is a BHF-funded Senior Research Fellow (Dept Haematology).
- Investigating CADASIL and CARASIL molecular mechanisms in young adult stroke Dr Alex Granata is a Medical Research Foundation Fellow and a Senior Research Associate (Dept Clinical Neurosciences)